Abstract
INTRODUCTION: Treatment outcomes of secondary Acute Myeloid Leukemia (sAML) including AML with myelodysplasia related changes (AML-MRC) and therapy related AML (tAML) are dismal compared to de novo AML patients, where long term disease free survival (DFS) remains less than 40%. Studies in pediatric AML identified frequent MYC somatic mutation and gene amplification, and although MYC somatic mutations are rare in adult AML, a recent study showed de novo AML patients expressing high levels of the MYC oncoprotein have inferior survival outcomes versus low levels of MYC. Compared to other AML subtypes, AML-MRC patients were shown to have dynamic range of MYC protein expression, yet the clinical significance of MYC levels in these patients group is unknown. Here we report the prognostic impact of MYC protein levels on survival outcomes in AML-MRC patients.
METHODS: Using Total Cancer Care (TCC) Moffitt Cancer Center (MCC) databases, we retrospectively identified histologically confirmed AML-MRC patients from 2011 to 2018. MYC protein expression was assessed by immunohistochemistry (IHC) staining. TP53 mutation was tested by 54 myeloid targeted gene sequencing. We used 5% as cut-off (calculated as MYC positive cells out of total counted blasts in the selected area with sheets of blasts) as previously reported (Ohanian et al. 2018). Clinical variables and disease-related prognostic factors including age, gender, cytogenetics and somatic mutations were characterized at the time of AML-MRC diagnosis and were annotated using descriptive statistics. The overall survival (OS) were estimated with the Kaplan-Meier method and compared using the log-rank test. All statistical analyses were performed using SPSS v24.0 and GraphPad Prism 7.
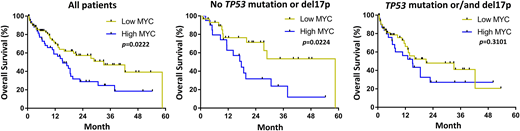
RESULTS: A total of 132 AML-MRC patients were included in this study. The median age at AML-MRC diagnosis was 67 (22-86) years and 64% of patients were male (n=84). A total of 49% (n=65) patients had chromosome 17p deletion [del(17p)] based on cytogenetic analyses or/and fluorescence in situ hybridization (FISH) assays. A total of 42% (n=55) patients had TP53 mutation and 29% (n=38) patients had both del(17p) and TP53 mutation. Additional chromosomal abnormalities including deletion 5q, trisomy 8, deletion 7q, deletion 20q, and complex karyotypes were observed in 28% (n=37), 17% (n=23), 20% (n=27), 7% (n=9), and 31% (n=41) of patients, respectively. A total of 55% (n=73) of patients were treated with intensive chemotherapy, 18% (n=24) were treated with hypomethylating agents and 20% (n=27) patients underwent allogeneic stem cell transplant. A total of 39% (n=51) patients had high MYC expression and 61% (n=81) patients had low MYC expression. Notably, the median OS was significantly longer in low MYC patients compared to high MYC patients (median OS 33.1 vs. 15.2 months, p=0.0222). Further, when considering only TP53 wild type patients without del(17p), low MYC patients had even longer median OS (median OS 58.6 vs. 17.7 months, p=0.0224). In AML-MRC patients with either TP53 mutation and/or del(17p), the median OS was not statistically different between low and high MYC groups (median OS 21.0 vs. 15.1 months, p=0.3101). Finally, multivariate analysis including TP53 mutation status, del(17p), transplantation status, gender, and age, revealed that high MYC expression is a poor prognostic factor (HR 2.08, 95%CI=1.136-3.807, p=0.018).
CONCLUSIONS: AML-MRC patients with high MYC expression have inferior OS outcome compared to low MYC patients. Further, multivariate analysis established that high MYC level is a poor prognostic factor in AML-MRC patients. These findings warrant further study of the prognostic impact of MYC expression in addition to MYC gene amplification or/and somatic mutations in AML patients, with larger numbers of patients having other somatic mutations or chromosomal abnormalities that have adverse outcomes.
Sallman:Celgene: Research Funding, Speakers Bureau. Sweet:BMS: Honoraria; Jazz: Speakers Bureau; Novartis: Consultancy, Honoraria, Speakers Bureau; Agios: Consultancy; Astellas: Consultancy; Agios: Consultancy; Phizer: Consultancy; Astellas: Consultancy; Celgene: Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria, Speakers Bureau; Celgene: Honoraria, Speakers Bureau; Jazz: Speakers Bureau; BMS: Honoraria; Phizer: Consultancy. Komrokji:Novartis: Honoraria, Speakers Bureau; Celgene: Honoraria, Research Funding; Novartis: Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau; Celgene: Honoraria, Research Funding; Novartis: Honoraria, Speakers Bureau. List:Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal